
Carbon monoxide (CO) and carbon dioxide (CO2) are often confused, but understanding their differences can be life-saving. While both are colorless, odorless gases, they behave very differently, pose different risks, and require specific detection methods to ensure safety in workplaces, homes, and industrial environments.
Bottom line - CO and CO2 Compared: What's the Difference?
- Carbon dioxide is a naturally occurring gas required for plant and animal life. Carbon monoxide is the result of incomplete combustion during burning.
- CO2 is a common, natural gas. CO is not.
- Fatalities from CO2 are rare. CO poisoning is common worldwide.
Table of Contents
- CO and CO2 – What’s the Same?
- CO and CO2 – What’s the difference?
- Is CO2 or CO more harmful?
- Why is CO more toxic than CO2?
- Do cars emit carbon monoxide or carbon dioxide?
- CO and CO2 Applications
- About CO2 - Carbon Dioxide
- CO2 Facts
- CO2 Recommended Limits
- About CO - Carbon Monoxide
- CO Facts
- CO Recommended Limits
- Understanding PPM - parts per million
- Best CO2 and CO Safety Monitors
CO and CO2 – What’s the same?
- Both are made from carbon and oxygen molecules
- Both are colorless, tasteless and odorless gases
- Both are in the air we breath (albeit in different concentrations)
- Both are released during combustion
- Both are important industrial gases
- Both are potentially deadly and can cause severe health problems
While both gases have the word "carbon" in their name, -monoxide (mono in Greek means 1) refers to the bond between a single carbon molecule and a single oxygen molecule while -dioxide (di in Greek means 2) refers to the bond between a single carbon molecule and two oxygen molecules, (oxide means a simple compound of oxygen). In other words, CO is C+O while CO2 is O+C+O.
Both carbon dioxide and carbon monoxide are colorless, odorless and tasteless gases. However, some describe the odor of high levels of CO2 as “acidic” or “bitter.”
While both CO and CO2 are potentially deadly, this happens at vastly different concentrations. While 35 ppm (0.4%) of CO is quickly life threatening, it takes more than 30,000 ppm (3%) of CO2 to reach the same risk level.
Compressed carbon dioxide and carbon monoxide are both important industrial gases. For example, CO2 is used to carbonate beverages and to increase plant growth in indoor greenhouses. CO is used during the manufacturing of iron and nickel as well as the production of methanol. In spite of their molecular similarity, they both behave very differently when interacting with other molecules.
CO and CO2 – What’s the difference?
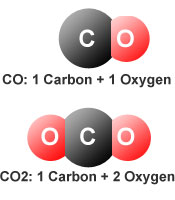
The most important difference is that carbon dioxide is a common, naturally occurring gas required for plant and animal life. CO is a byproduct of the burning of fossil fuels such as oil, coal, and gas.
CO poisoning occurs when carbon monoxide builds up in your bloodstream. Your body replaces the oxygen in your red blood cells with carbon monoxide. leading to serious tissue damage. CO2 poisoning occurs when the lungs cannot take in enough oxygen.
CO2 does not undergo oxidation reactions and is a non-flammable gas. In comparison, CO undergoes oxidation reactions and is classified as a flammable gas.
- CO2 has a molar mass of about 44g/mol.
- CO has a molar mass of about 28g/mol.
Another general difference is the number of carbon and oxygen atoms. Carbon Monoxide contains one carbon and one oxygen atom, whereas carbon dioxide contains one carbon and two oxygen atoms.
Use this quick and easy reference chart to gain a better understanding:
| Aspect | CO (Carbon Monoxide) | CO2 (Carbon Dioxide) |
|---|---|---|
| Formula | CO | CO₂ |
| Natural Occurrence | Rare, man-made | Naturally occurring |
| Toxicity | Highly toxic at low ppm | Toxic only at high ppm |
| Flammability | Flammable | Non-flammable |
| Health Impact | Binds to hemoglobin, prevents O2 transport | Displaces oxygen at high concentrations |
| Typical Sources | Incomplete combustion | Respiration, combustion |
Is CO2 or CO more harmful?
Both carbon dioxide (CO2) and carbon monoxide (CO) are very harmful when present in high concentrations, however, they do hold different levels of toxicity and effects when it comes to the environment and human health.
Carbon Dioxide Gas
Carbon dioxide is a naturally occurring gas and a significant component of Earth's atmosphere. It is produced through natural processes, such as respiration and volcanic activity, and is also a byproduct of human activities, especially the burning of fossil fuels (e.g., coal, oil, and natural gas) for energy.
- Environmental impact: Carbon dioxide is a greenhouse gas, which means it traps heat in the Earth's atmosphere, leading to the greenhouse effect. While it is necessary for maintaining Earth's temperature and supporting life, excessive CO2 emissions from human activities have led to an enhanced greenhouse effect, resulting in global warming and climate change. This contributes to issues such as rising sea levels, extreme weather events, and even disruptions to our ecosystems.
- Human health impact: Carbon dioxide is generally not toxic to humans in the concentrations typically found in the atmosphere (approximately 0.04% by volume). However, in enclosed or poorly ventilated spaces, high concentrations of CO2 can displace oxygen and cause suffocation, asphyxiation, and even fatalities.
Carbon Monoxide Gas
Carbon monoxide is a colorless, odorless, and tasteless gas produced primarily by incomplete combustion of carbon-containing fuels. Common sources include vehicle exhaust, malfunctioning home heating systems, and fires.
- Environmental impact: Carbon monoxide is not a significant greenhouse gas and does not contribute directly to global warming like carbon dioxide. However, it can indirectly affect the environment by contributing to the production of ground-level ozone, which is also harmful to both human health and the process of vegetation.
- Human health impact: Carbon monoxide is highly toxic to humans. When inhaled, it binds to hemoglobin in the blood, reducing its ability to carry oxygen, which can lead to carbon monoxide poisoning. Symptoms of CO poisoning include headaches, dizziness, weakness, nausea, and confusion. High concentrations can be fatal. Because carbon monoxide is odorless and colorless, it is particularly dangerous in enclosed spaces without proper ventilation.
Both carbon dioxide and carbon monoxide have their risks and dangers, especially in confined spaces. It is crucial to address both of these differently.
Why is CO more toxic than CO2?
While both carbon dioxide and carbon monoxide are gases that can pose health risks, they each have different effects on the body.
Carbon Dioxide Toxicity
When an individual inhales or breathes in high concentrations of CO2, it can lead to hypercapnia, a condition characterized by elevated levels of carbon dioxide in the bloodstream. Symptoms may include headaches, dizziness, shortness of breath, confusion, and in severe cases, unconsciousness or death.
When we look at long-term exposure, chronic exposure to elevated levels of CO2 can lead to respiratory acidosis, a condition where the lungs cannot remove enough carbon dioxide, resulting in an imbalance of acidity in the blood. This can lead to fatigue, confusion, and eventually, organ damage.
Carbon Monoxide Toxicity
Alternatively, carbon monoxide is highly toxic, as it binds to hemoglobin in the blood more strongly than oxygen does, leading to reduced oxygen delivery to tissues and organs. Symptoms of CO poisoning include headache, dizziness, nausea, confusion, weakness, and in severe cases, loss of consciousness or death.
Prolonged exposure to low levels of CO can cause chronic health problems, including cardiovascular issues and neurological damage. Pregnant women, infants, and individuals with cardiovascular diseases are also particularly vulnerable.
While both CO2 and CO can pose severe health risks, carbon monoxide is generally considered more immediately toxic at lower concentrations, while high levels of carbon dioxide primarily lead to respiratory problems. However, both gases can be dangerous if not properly monitored and proper ventilation and is essential to minimize exposure and mitigate risks.
Do cars emit carbon monoxide or carbon dioxide?
Cars emit both gases. Carbon dioxide is the result of complete fuel combustion, while some carbon monoxide is emitted from incomplete combustion.
Newer vehicles emissions systems keep carbon monoxide levels low. However, CO from older cars (pre-catalytic converter) or poorly tuned cars can increase the risk of hazard if this system isn't working properly.
However, in an enclosed garage, you can get deadly levels of carbon monoxide from a gas vehicle at idle. This is why many garages and parking ramps have CO detectors.
CO and CO2 Applications
Both carbon dioxide and carbon monoxide are commonly used gases in various applications and industries. Below, we highlight the main applications the gases can be found in.
Carbon dioxide
- Indoor Agriculture - growing plants
- Beverage Dispensing - CO2 is the "fizz" in soda and beer
- Welding, Industrial - MIG welding shield gas
- Refrigerant, Fire Suppression
- Pharmaceutical and Laboratory
Carbon monoxide
- Hydrogen Production
- Petrochemical
- Food Processing - makes packaged meat "red"
- Metallurgy - reduce pure metals from ores
About CO2 - Carbon Dioxide
Carbon dioxide (CO2) is a colorless, odorless and tasteless gas. It is nonflammable at room temperature. The linear molecule of a carbon atom that is doubly bonded to two oxygen atoms, O=C=O.
Where does CO2 come from?
CO2 is a naturally occurring gas in earth's atmosphere. It is naturally produced by the decomposition of organic matter. It is also naturally produced by animal and human respiration, which takes in oxygen and exhales CO2. Plants and trees depend on CO2 for life (they take in CO2 and give out oxygen).
Carbon dioxide can also be produced through industrial processes. For example, industrial plants that produce hydrogen or ammonia from natural gases are some of the largest commercial producers of carbon dioxide.
Solid carbon dioxide is also known as "dry ice." An interesting fact about CO2 is that it coverts directly from a solid to a gas at -78°C or above.
While not as deadly as carbon monoxide, high levels of CO2 in an enclosed space – for example, in a submarine – can suffocate you long before the oxygen runs out. In fact, dozens of people die or are injured each year as the result of CO2 leaks in bars, restaurants or in unventilated keg coolers when a beer line is left open. Others die in dry ice (frozen carbon dioxide) storage lockers used for temporary food storage.
For protection from CO2 in enclosed spaces, CO2Meter offers CO2 safety alarms.
CO2 Facts
- CO2 is a common gas in the atmosphere and is required for plant life
- CO2 is a natural byproduct of human and animal respiration, fermentation, chemical reactions, and the decomposition of plant and animal life.
- In the atmosphere CO2 measures approximately 400 ppm (parts per million).
- CO2 is non-flammable, with no explosive properties
- CO2 poisoning is rare; however scuba divers have to watch out for it (the bends)
- Leaking pressurized CO2 tanks in enclosed areas can be dangerous for occupants - both from high levels of CO2 and from lower levels of oxygen (See oxygen displacement / asphyxiation).
CO2 Recommended Limits
- 410 ppm is the current average CO2 level on the planet
- ASHRAE recommends a 1,000 ppm limit for office buildings and classrooms to ensure overall health and performance
- OSHA limits workplace exposure levels to 5,000 ppm time-weighted average (over 8 hours)
- Drowsiness can occur at 10,000 ppm (1%) – common in closed cars or auditoriums
- Symptoms of mild CO2 poisoning include headaches and dizziness at concentrations less than 30,000 ppm (3%)
- At 40,000 ppm (4%) or above CO2 can be life-threatening
See our carbon dioxide levels chart here.
About CO - Carbon Monoxide
Like carbon dioxide, carbon monoxide is also a colorless, odorless, and tasteless gas - that is toxic and has the molecular formula CO. Many refer to carbon monoxide (CO) as one of the most dangerous gases.
Where does CO come from?
Carbon monoxide is the result of incomplete combustion. This happens when there is a limited supply of oxygen available.
While not normally occurring in nature, CO is a commercially important chemical, and is the result of oxygen-starved combustion from improperly ventilated fuel-burning motors and appliances like:
- Oil and gas furnaces
- Gas water heaters or gas ovens
- Gas or kerosene space heaters
- Fireplaces and wood stoves
- Portable generators
- Cars with or without catalytic converters
Too much carbon monoxide in an unventilated space is deadly.
In fact, carbon monoxide poisoning is the most common type of fatal poisoning worldwide. This is why many new homes are built with CO detectors in addition to smoke detectors.
CO Facts
- CO is almost entirely a man-made gas that is not normally found in the earth's atmosphere.
- CO is produced at dangerous levels by oxygen-starved combustion in improperly ventilated fuel-burning appliances such as generators, oil and gas furnaces, gas water heaters, gas ovens, gas or kerosene space heaters, fireplaces, and stoves
- The highest CO emissions are produced by internal combustion engines without a catalytic converter.
- CO can be a flammable gas in higher concentrations (sometimes referred to as C1D1 or C2D2 environments). Devices to measure carbon monoxide in these concentrations are often designed to be explosion-proof.
- CO is the most common type of fatal poisoning in the world.
CO Recommended Limits
- Symptoms of mild CO poisoning include headaches, dizziness, and violent vomiting at concentrations less than 100 ppm
- 0.1 ppm is the current average CO level on the planet
- 9-50 ppm is the standard maximum limit for an 8-hour workday
- 200-400ppm will result in physical symptoms followed by unconsciousness and death within hours
- Concentrations above 800 ppm can be life-threatening in minutes
See our carbon monoxide safety levels chart here.
Understanding PPM - parts per million
While large gas concentrations in a volume of air are measured in percentages, small volumes are measured in parts-per-million - ppm.
When measuring small volumes, the range of concentrations is from 0 to 1,000,000, which equals 0-100%. Every 10,000 ppm equals 1% concentration. For example, instead of saying "1% gas by volume," scientists will say "10,000 ppm." This is because 10,000 / 1,000,000 = 1%.
Read more about parts-per-million here.
Choosing the Right CO2 vs. CO Gas Detection Monitor
When it comes to gas safety, selecting the right gas detector is critical for ensuring a safe environment in workplaces, restaurants, breweries, laboratories, and other industries that use or store carbon dioxide (CO2) and carbon monoxide (CO).
The right gas detection safety device should be accurate, reliable, and compliant with safety regulations, helping to protect employees and customers from potentially hazardous gas exposure.
CO2 Safety: Remote CO2 Storage Safety 3 Alarm (RAD-0102-6)

For businesses handling bulk CO2 storage, such as beverage facilities, restaurants, and breweries, the Remote CO2 Storage Safety 3 Alarm (RAD-0102-6-HS2) is an essential fixed gas detector. This device is designed to monitor CO2 levels and alert users before concentrations reach dangerous levels, ensuring compliance with fire and safety codes like NFPA, OSHA, and IFC requirements.
Key Features:
- Continuous CO2 monitoring with real-time digital display
- Three alarm levels with audible and visual alerts for safety compliance
- Remote sensor unit to allow for flexible placement in confined spaces
- Included Horn Strobes for audible/visual alarm indication
- Meets regulatory requirements for CO2 safety monitoring in storage areas
For additional carbon dioxide safety monitoring solutions, visit our website.
CO Safety: CO2Meter Fixed Carbon Monoxide Gas Detector
For environments where carbon monoxide exposure is a risk—such as manufacturing plants, parking garages, or industrial facilities—the Fixed Carbon Monoxide Gas Detector provides reliable protection. This device can monitor 1-3 different gases simultaneously, making it a versatile solution for facilities that need multi-gas detection.
Key Features:
- Capable of detecting CO alone or in combination with other gases
- Real-time monitoring with adjustable alarm thresholds
- Easy integration with existing safety systems for added protection
- Ideal for enclosed spaces where CO exposure poses a serious risk
Choosing the Right Gas Detector
When selecting a CO2 or CO safety monitor, consider the following:
- Industry Regulations: Ensure compliance with OSHA, IFC, and local fire codes.
- Fixed vs. Portable Needs: Fixed monitors like the RAD-0102-6 and CO2Meter Fixed CO Detector provide continuous safety monitoring in high-risk areas.
- Multi-Gas Capabilities: If multiple gases need monitoring, opt for a multi-gas detector for broader protection.
For businesses prioritizing comprehensive gas safety, investing in high-quality fixed gas detectors like CO2Meter’s solutions ensures compliance, workplace safety, and peace of mind.
Learn more about our carbon dioxide and carbon monoxide detectors.







